|
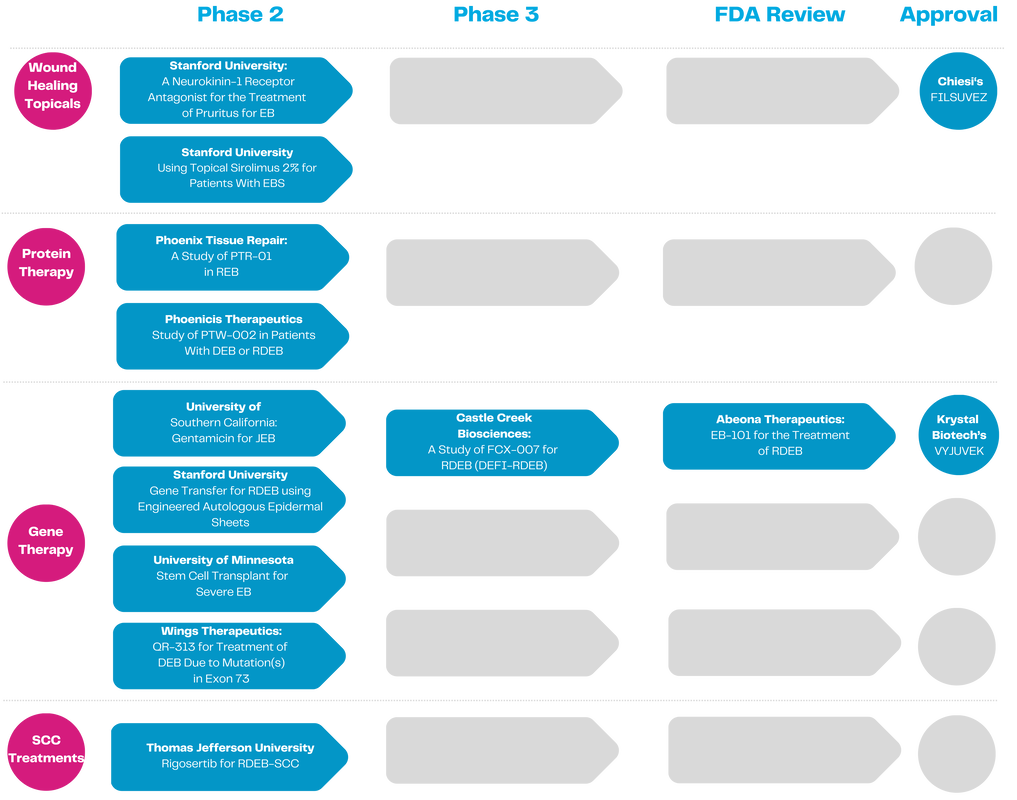
Over the last decade, EBRP has made remarkable strides, including helping to accelerate the first two EB treatments FDA approved in 2023.
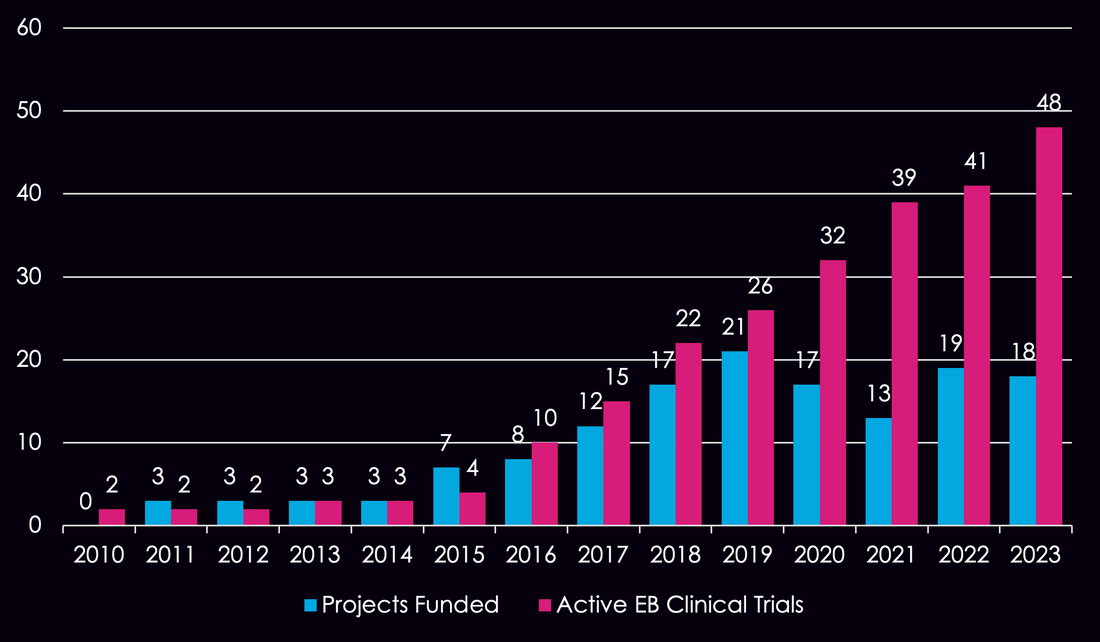
Since our founding, we have funded 140 research projects laser-focused on bringing life-saving therapies and cures to the EB community. Our work has transformed the clinical trial landscape from just 2 clinical trials in EB to over 40 today, an almost 20x increase.
|
|
"Adapting the iPS Cell-Based Therapy for RDEB to an Automated Platform to Facilitate Clinical Translation" |
"Suppressing the Itch of Dystrophic Epidermolysis Bullosa" |
"Development of a Novel Ophthalmic Solution to Treat Ocular Manifestations of RDEB" |
|
2023
18 PROJECTS APPROVED $5.9M AWARDED |
2022
19 PROJECTS APPROVED $6.6M AWARDED |
|
2021
13 PROJECTS APPROVED $5.2M AWARDED 2019
21 PROJECTS APPROVED $7.5M AWARDED |
2020
17 PROJECTS APPROVED $7.2M AWARDED 2018
17 PROJECTS APPROVED $11M AWARDED |
|
21 INSTITUTIONS, ONE FOCUS: CURING EB
Collaboration is central to our model. The EB Clinical Research Consortium (EBCRC) allows each research team to benefit from the others, accelerating progress to heal EB. |
DRIVING RESULTS FOR PATIENTS.
We find the most promising research projects to fund. But we don't just sign a check and hope for the best. Our innovative Venture Into Cures system ensures maximum impact for the EB community. |